Personal Science Week - 251023 Complementary Science
Sometimes bad science isn't wrong
We’ve been busy in Austin this week at a biohacking event that we’ll discuss in future posts.
Meanwhile, we’re revisiting and expanding a post from PSWeek221013 about philosopher Hasok Chang’s concept of “complementary science”—an idea that’s become increasingly relevant as we think about what gets lost when scientific knowledge becomes oversimplified.
Thermometers have been around for a long time – Galileo had one in 1600. So how come even a genius like Isaac Newton didn’t know that water has a constant boiling temperature? He actually thought it had a range of values, distinguishing between the temperature at which water “begins to boyle” and when it “boyles vehemently.”
Even the idea that water boils at a specific temperature is easily disproved by simply watching a tea pot: clear bubbles form well under 100ºC, making it hard to describe exactly when the “boiling” begins. And unless you’re specifically thinking that altitude might make a difference, somebody in a mountain town can prove you wrong about any claim you make at sea level.
The Lost Science of Boiling Water
But Newton wasn’t wrong – or at least, not entirely. In fact, around 1800, many reputable scientists reported significant variations in the boiling temperature of pure water under normal atmospheric pressure. French physicist Jean-Baptiste Biot observed that water boiled at different temperatures in glass versus metal containers – a difference of over 1°C. Swiss physicist Jean-André De Luc reported even more dramatic results: by extracting dissolved air from water, he found he could “superheat” it to 112°C before it boiled.
These weren’t fringe researchers or sloppy experimentalists. In 1776, the Royal Society even appointed a formal committee to investigate and establish the true boiling point of water. The phenomenon was widely known and actively researched throughout the 18th and 19th centuries.
Then something curious happened: all this knowledge disappeared. With the development of modern thermodynamic theory, these idiosyncrasies were collapsed into a single boiling point of 100°C, and the rich experimental tradition was essentially forgotten. Today’s chemistry textbooks confidently state that pure water boils at 100°C at standard pressure, full stop.
Testing History in the Lab
University of Cambridge Professor Hasok Chang faced what he calls a “problem of incredulity” when he encountered these historical claims. Were the 18th and 19th-century scientists right? Or was this an error, like the infamous cases of “cold fusion” or “N-rays”? He decided there was only one way to find out: replicate the experiments himself.
Chang went into the lab and systematically reproduced—and confirmed!— the historical experiments. Water heated in a metal vessel struggled to reach 99°C, while water heated very slowly could reach 104°C. When he replicated De Luc’s degassing method, he observed boiling temperatures creeping well above 100°C – even when using anti-bumping granules that are supposed to promote smooth boiling at the standard temperature.
What Chang discovered is that water boils at the “boiling point” only under very particular circumstances. Our common-sense intuition about the fixedness of the boiling point is sustained only by our limited experience – we typically boil water in the same types of vessels, with similar amounts of dissolved air, under similar conditions.
The Tragedy of Everyday Science
Chang uses these findings to illustrate what he calls the “tragedy of everyday science”: cutting-edge science is not necessarily the best for all purposes. Consider batteries, which were critical to the development of electrical circuits and fundamental theories like Ohm’s Law. Today, chemistry students learn about the obsolete 19th-century “Daniell cell” because the chemistry behind modern batteries is too complicated to explain except to specialists. Even the advanced graduate-level textbooks Chang consulted gave the wrong explanation.
Science is full of such examples – facts we take for granted that were actually spectacular achievements, obtained only after innovative thinking, painstaking experiments, bold conjectures, and controversy. More importantly, in the process of scientific specialization, certain ideas and questions get suppressed if they contradict or destabilize knowledge that needs to be taken for granted in day-to-day work.
Complementary Science as a Discipline
Chang proposes “complementary science” as a way to fill in these cracks of important and useful knowledge too often left behind. It requires an interest in both history and philosophy, reflecting on questions that specialists lack the luxury to pursue:
Why and how did specialists emerge to pursue this particular direction and not another?
Were other, promising routes abandoned prematurely?
What experimental facts, once well-established, have been forgotten?
This isn’t just an academic exercise. Complementary science can enrich the factual basis of science teaching, improve our understanding of the nature of science, foster habits of original and critical inquiry, and attract students through a renewed sense of wonder. When Chang demonstrated superheating experiments to students, watching the thermometer creep eerily above 100°C as water boiled, it challenged their fundamental assumptions about what they “knew” to be true.
Beyond Boiling Water: The Personal in Science
The same pattern Chang observed with water’s boiling point appears throughout medicine. We all “know” that normal body temperature is 98.6°F, but modern studies show only 8% of healthy people actually have that exact temperature. Your personal baseline might be 97.2°F or 99.1°F—and it can vary by nearly a full degree throughout the day. Similarly, “ideal weight” standards from 1958 look dramatically different from today’s ranges. Are we healthier now, or were people then? The answer depends on which metrics you prioritize.
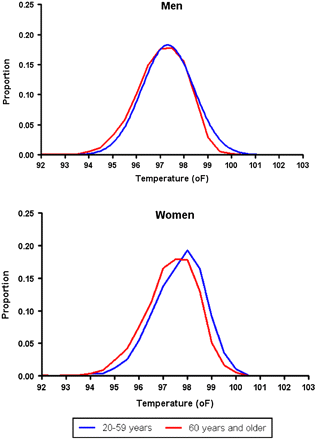
(We explored these issues in detail in our posts on what’s normal and fitness comparisons.)
This is where Chang’s “complementary science” becomes intensely practical. Modern medicine, like modern thermodynamics, has simplified many measurements into single numbers that work well enough for most purposes—but that lose critical information in the process. Just as water doesn’t actually boil at exactly 100°C under all conditions, your body doesn’t conform to textbook “normals.” Don’t assume the “medically-correct” answer is actually correct for your own life.
Learning from History
For an introduction to Chang’s ideas, the 30-minute YouTube lecture “The Challenge of Everyday Science” provides an excellent overview. His books Inventing Temperature: Measurement and Scientific Progress and Is Water H2O?: Evidence, Realism and Pluralism offer deeper dives into how seemingly simple questions reveal profound complexities about scientific knowledge and practice.
We need specialists who dig deeply into particular fields – that’s undeniable. But complementary science reminds us that the cutting edge isn’t all there is to science, and that looking backward can sometimes point the way forward.
About Personal Science
Personal scientists apply the principles of scientific inquiry to understand our own lives—tracking our own baselines, running our own experiments, and questioning received wisdom that may not apply to us as individuals. We’re skeptical of oversimplified “normals” and population averages, preferring to discover what actually works for our unique circumstances.
We publish Personal Science every Thursday for anyone who wants to use science for personal rather than professional reasons. If you have topics you’d like us to explore, let us know.


